Aluminum is one of the most important metals on our planet. It is used in various applications, from food and beverage cans to cars and airplanes. But where does aluminum come from? The answer lies in bauxite, an ore that contains large amounts of aluminium oxide. Read on to learn more about the extraction process of aluminum from bauxite.
Bauxite is a mineral that contains various elements such as aluminium, silica, and iron oxide. The extraction of aluminium from bauxite begins with the crushing of bauxite ore into small particles. After this initial step, bauxite is leached with a hot caustic soda solution in order to separate the alumina and other impurities. The next step involves subjecting the leached product to electrolysis where electricity is used to extract pure aluminium. In conclusion, these steps lead to the successful extraction of aluminium from bauxite ore which makes it possible for us to use it in numerous applications like vehicles and electronic components.
Extraction Process
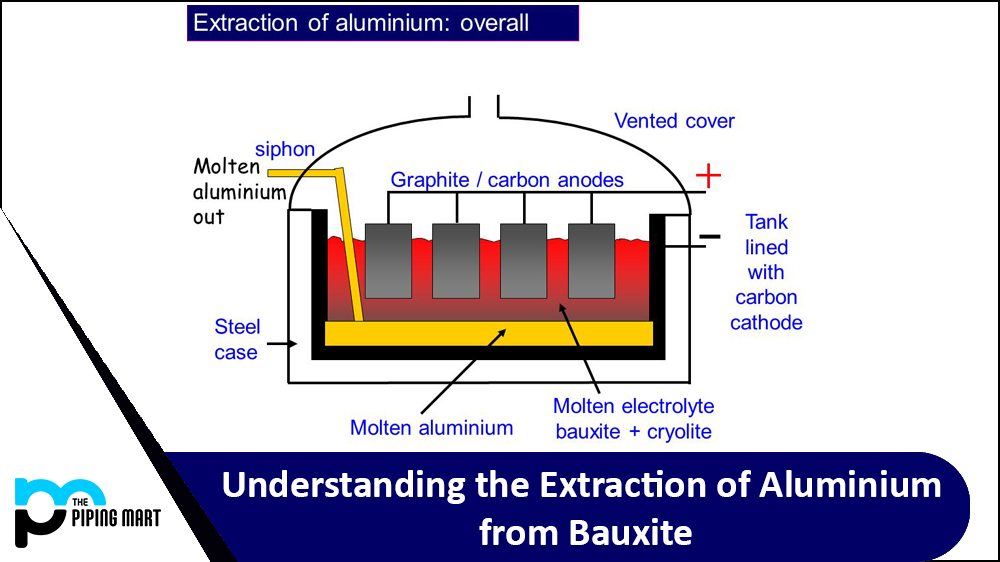
Bauxite is heated with a mixture of sodium hydroxide and calcium oxide at temperatures ranging from 700°C to 900°C. This results in a highly caustic liquor known as “red mud,” which contains iron, silicon and titanium oxides. The next step is precipitation, which involves adding cold water to the red mud slurry. This causes aluminum hydroxide (Al(OH)3) to form and settle out while impurities are left behind in the solution. The resulting sludge is then filtered off and calcined (roasted) at temperatures up to 1000°C to produce pure alumina (Al2O3).
The alumina is then dissolved into molten cryolite (Na3AlF6) at 980°C, forming a solution known as aluminum fluoride electrolyte. An electric current is passed through this electrolyte solution, causing aluminum atoms to be extracted from their ionic bonds with oxygen atoms within the alumina molecules. These removed aluminum atoms then bond together to form solid aluminum metal, which can be collected or further processed for specific uses.
Conclusion
In summary, extracting aluminium from bauxite involves heating bauxite with sodium hydroxide and calcium oxide before adding cold water to create aluminum hydroxide sludge. This sludge is then filtered off and calcined at high temperatures before being dissolved into molten cryolite to extract out the aluminum atoms using electricity. With this understanding of how aluminium is extracted from bauxite, you can now appreciate just how impressive this process truly is!

Pipingmart is B2B portal specializes in industrial, metal and piping products. Also, share latest information and news related to products, materials and different types grades to help business dealing in this industry.