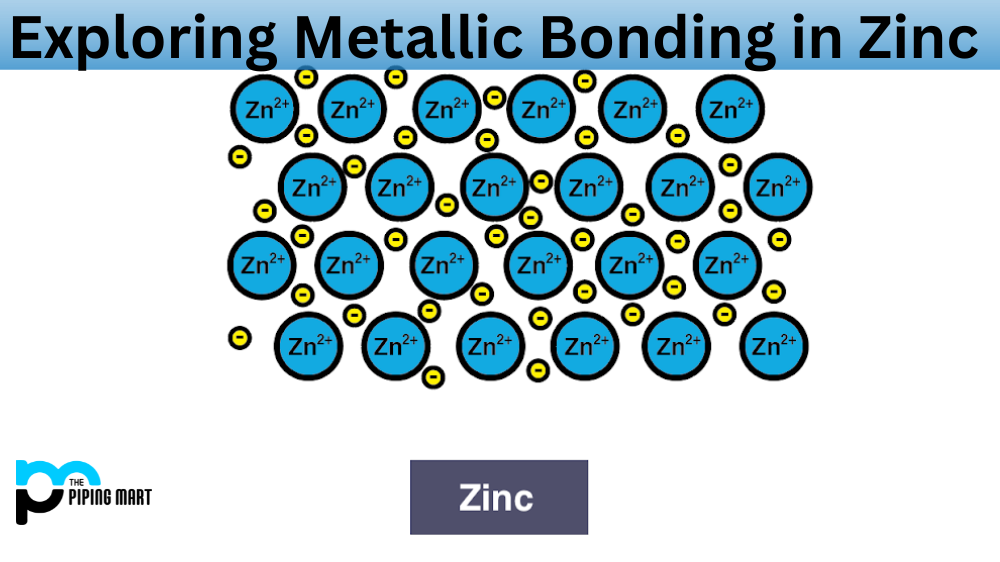
Have you ever wondered how zinc molecules bond together to create a solid metal? It all comes down to the science of metallic bonding, which is the force that holds atoms together in a metal structure. In this blog post, we’ll take a closer look at the process of metallic bonding in zinc and why it’s so important.
What is Metallic Bonding?
Metallic bonding is an atomic-level phenomenon that occurs when positively charged metal ions are held together by a cloud of electrons. This type of bond is responsible for the strength and malleability of many metals, including zinc. When two or more zinc atoms come into contact with one another, their valence electrons are shared. This forms a strong bond between the two atoms, creating what’s known as a lattice structure. The lattice structure is held together by strong covalent bonds between the atoms’ shared electrons and nuclei.
Why Is Metallic Bonding Important for Zinc?
Metallic bonding plays an important role in giving zinc its distinct properties. For starters, it gives zinc its strength and malleability—essential characteristics for any metal used in construction or other applications where strength and durability are essential. Furthermore, metallic bonding makes zinc resistant to corrosion and oxidation—two common issues that can be detrimental to materials exposed to moisture or oxygen over time. It also helps make zinc an excellent electrical conductor, which makes it useful in electronic applications such as batteries or circuit boards. Finally, metallic bonding allows zinc to be molded into various shapes when heated up—a useful property for manufacturing parts out of this material.
Conclusion:
Metallic bonding plays an essential role in making zinc one of the most versatile metals on Earth. From construction to electronics and beyond, metallic bonding gives zinc its unique set of properties that make it ideal for various applications. Next time you see something made from zinc, remember that it’s not just the element itself but its chemical structure that makes it so special!

A passionate metal industry expert and blogger. With over 5 years of experience in the field, Palak brings a wealth of knowledge and insight to her writing. Whether discussing the latest trends in the metal industry or sharing tips, she is dedicated to helping others succeed in the metal industry.