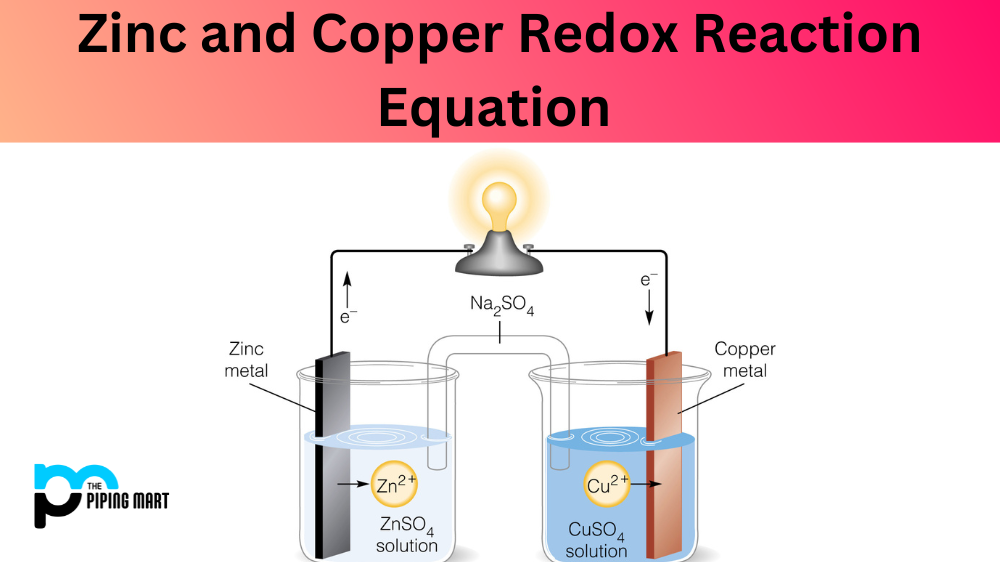
When two elements, such as zinc and copper, interact with one another in a chemical reaction, it is known as a redox reaction. A redox reaction occurs when electrons are shared between atoms in order to form new compounds. This type of reaction can be represented by an equation that shows how the elements interact. Let’s take a look at the zinc and copper redox reaction equation and what it means for us.
Zinc and Copper Redox Reaction Equation
The zinc and copper redox equation is written as follows:
2Zn + Cu2+ → 2Zn2+ + Cu.
In this equation, zinc (Zn) is oxidized while copper (Cu) is reduced. The subscript “2” indicates there are two of each element being used in the reaction. Oxidation is when an element loses electrons, while reduction is when an element gains electrons. You can tell which elements are oxidized or reduced by looking at the sign before them; if there is a “+” sign, then it is being oxidized, while a “-” sign indicates that it is being reduced.
Equation Significance
The zinc and copper redox reaction equation illustrates how these two elements interact with one another to form new compounds. This equation helps us understand why certain reactions occur, which can be useful for both scientific and industrial applications. For example, understanding this equation could help scientists develop new materials or create more efficient energy sources using these two elements. Additionally, industries such as mining may use this information to find better ways to extract metals from ore deposits efficiently or create more effective alloys for manufacturing purposes.
Conclusion
Overall, the zinc and copper redox reaction equation demonstrates how these two elements interact with one another during a chemical reaction to form new compounds. By understanding this equation, we can gain insight into why certain reactions occur and apply this knowledge to develop new materials or create more efficient energy sources for both scientific and industrial applications. With further research into oxidation-reduction reactions, we may uncover even more ways in which these reactions can be utilized in our everyday lives!

Pipingmart is a B2B portal that specializes in metal, industrial and piping items. Additionally, we share the latest information and information about materials, products and various types of grades to assist businesses that are involved in this business.