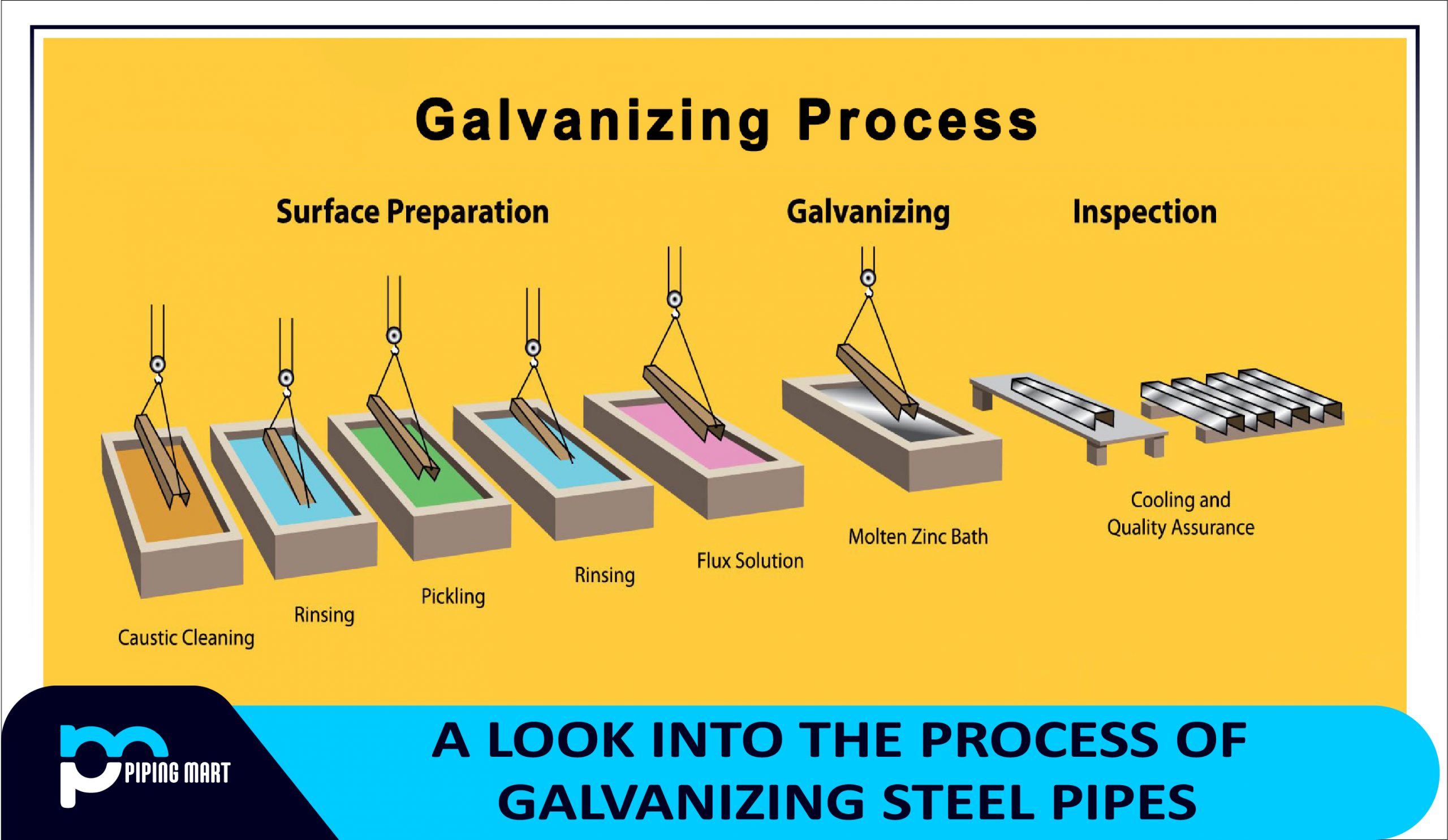
- Metal Preparation
- Dipping Phase into Molten Zinc
- Cooling, Finishing, and Drying
- Quality Control
Galvanized iron pipes are widely used in a variety of useful applications, including water transportation, farming, telecommunication, and plumbing. Galvanized iron pipes are excellent for both indoor and outdoor use due to their corrosion resistance and longevity. But have you ever wondered how these things are made? The galvanizing procedure of these steel pipes is what makes them so robust and desirable for engineering and building projects all over the world. Today, we’ll be discussing the same! A deeper look into the process of making galvanized iron pipes is described here.
Metal Preparation
Before anything else, the iron pipes are meticulously prepared on the surface. Before the base metal can be galvanized, it must go through a variety of processes. All of this ensures that the iron is ready for the next stage. Typically, the surface preparation of galvanized iron pipes works as follows:
- First, the steel is cleaned with a chemical solution, which is a corrosive material that removes all oil, grease, and dust.
- Afterward, when the chemical solution has been washed away, the iron pipe is pickled. Pickling is a metal surface process that removes stains, contaminants, pollutants, rust, and scale from ferrous metals.
- Pickling the iron pipe in an acidic solution removes mill scale, commonly known as the flaky surface of hot-rolled steel.
- The iron pipe will not be fluxed once the pickling solution has been washed away. To prevent oxidation of the cleaned surface, zinc ammonium chloride is commonly applied to the base metal.
Dipping Phase into Molten Zinc
Although all stages of the galvanizing process are essential, this phase and approach are what make it so popular. The iron pipes are extensively submerged in a bath of molten zinc that is about 449 degrees Celsius, or 840 degrees Fahrenheit. It will create a thick and strong coating on the surface of the base metal here.
Why is zinc used? Zinc is the substance that prevents oxygen and water from passing through the base metal underneath. It also contributes significantly to the metal’s resistance to rusting and corrosion. When the outer layer is contacted by dust and friction, zinc acts as a sacrificial anode.
Some manufacturers add lead to the molten zinc bath to make it more liquid and to cap the amount of zinc on the dipped iron pipe. This also aids in the prevention of floating dross.
Some producers may use cold-dipped galvanizing, in which the base metal is simply sprayed with zinc-rich paint. However, hot-dipped galvanizing is the most commonly used process presently. Since 1742, it has been shown to give a better, longer-lasting, and maintenance-free coating for metals.
Cooling, Finishing, and Drying
Once the zinc dipping procedure has been completed, it is now time for the galvanized iron pipes to cool. Typically, galvanized iron pipes are cooled in a quench tank to allow them to cool fast and prevent undesired effects of the freshly formed coating from reacting with the environment of their surroundings.
Quality Control
Quality standards are strictly enforced on all manufactured products. The galvanized iron pipes are tested here to see if they meet the final product criteria.
Quality control is not one-size-fits-all, since each manufacturer has its approaches and techniques for assuring the quality and standards of galvanized iron pipes.

Pipingmart is B2B portal specializes in industrial, metal and piping products. Also, share latest information and news related to products, materials and different types grades to help business dealing in this industry.