What is the passivation process?
By eliminating ferrous impurities like free iron from their surface, the passivation process is a technique for enhancing the corrosion resistance of stainless steel parts and returning them to their original corrosion standards. Rust is resisted by passivated stainless steel.
ASTM A380 states that passivation is –
“removal of exogenous iron or iron compounds from the surface of a stainless steel by means of a chemical dissolution, most typically by a treatment with an acid solution that will remove the surface contamination but will not significantly affect the stainless steel itself … for the purpose of enhancing the spontaneous formation of the protective passive film.”
It is a chemical process that improves the ability of stainless steel and other alloys to resist corrosion. Passivated machinery and systems have numerous advantages:
- Surface contamination is eliminated via passivation.
- Increased corrosion resistance after passivation
- The possibility of product contamination is decreased through passivation.
- You can extend system maintenance windows with passivation.
- Extended life of the product
How does passivation work?
Iron, nickel, and chromium are the three main elements found in most stainless steel alloys. The chromium concentration in stainless steel is what gives it its resistance to corrosion. When chromium is in contact with oxygen (air), chromium oxide is created, covering the stainless steel surface and preventing the iron underneath from rusting. To enhance and improve the production of the chromium oxide layer is the goal of passivation.
When stainless steel is immersed in an acid bath, the surface-free iron is dissolved but the chromium is left unaltered. Chemical action of the acid causes the free iron to be chemically removed, leaving an uniform surface with more chromium than the underlying substance. The stainless steel generates the chromic oxide layer over the following 24 to 48 hours after being exposed to oxygen in the air after the acid bath. In order to create a thicker, more protective coating of chromium oxide, a larger proportion of chromium must be present at the surface. Corrosion cannot begin if free iron is removed from the surface. A chemically inert surface that prevents rust is provided by the resulting passive layer.
When is the passivation of stainless steel required?
After grinding, welding, cutting, and other machining operations that alter stainless steel, passivation is a post-fabrication procedure that is carried out. Stainless steel naturally resists corrosion under ideal conditions, which may imply that passivating wouldn’t be necessary. However, any of the following can prevent the production of the oxide film that prevents corrosion under typical, actual circumstances:
- an industrial environment with foreign materials (shop dirt, grinding swarf)
- stainless steel with sulfides added for better machinability
- The surface of stainless steel parts has iron particles from cutting tools.
To restore a uniformly corrosion-resistant surface, such impurities must be eliminated right down to the surface grain boundaries. These problems are fixed through passivation.
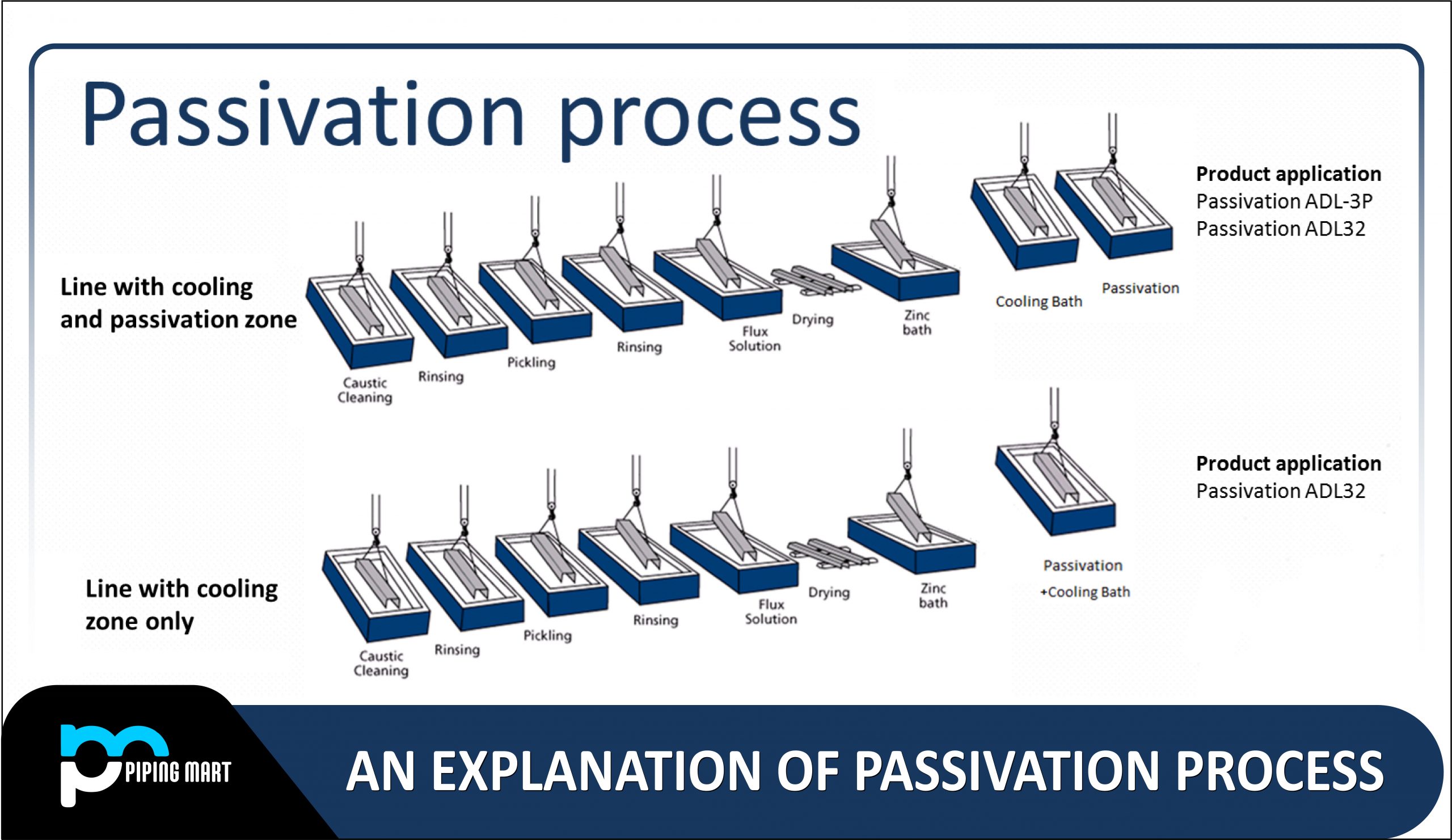
How is it done?
Two steps make up the procedure. The first step is to completely clean the component to remove any contaminants that were applied to its surface during the manufacturing process. Since contamination might harm the metal, this stage is crucial to the process.
The steel must then go through a passivating bath in the following phase. For the acidic bath, three methods can be applied:
- Nitric acid passivation
- Nitric acid with sodium dichromate passivation
- Citric acid passivation
Methods of passivation :-
- Gel Application: Gels or pastes can be applied manually by brushing them on the surface. It is helpful for hand-detailed spot treatment of welds and other delicate areas.
- Circulation: A chemical solution is moved via a circulation of pipes, and this method is especially advised for piping that will convey corrosive liquids.
- Tank Immersion: This method is beneficial for treating all the fabrication surfaces at once for finish uniformity and the best corrosion resistance.
- Spray Application: This method of on-site treatment has advantages, but it must be used with caution and according to the right safety precautions.
Thus , the creation of a passive layer that does not easily interact with the environment is the primary goal of passivation treatment.

Pipingmart is B2B portal specializes in industrial, metal and piping products. Also, share latest information and news related to products, materials and different types grades to help business dealing in this industry.