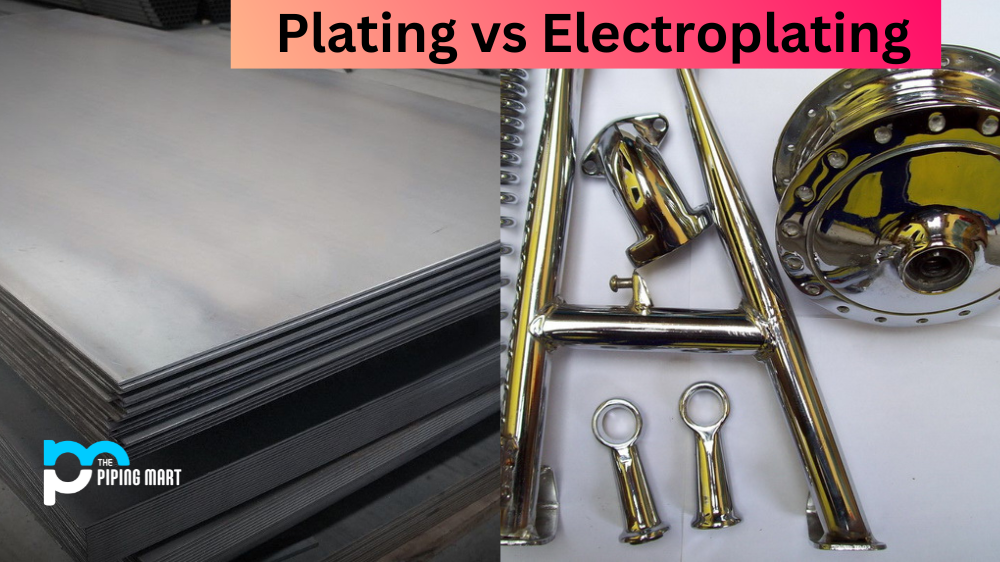
Both plating and electroplating are processes used to coat objects with a layer of metal. This coating technique can enhance the appearance of an object and also help protect it from corrosion, humidity, and other environmental factors. But what is the difference between plating and electroplating? Let’s take a closer look at both processes to understand how they differ.
Plating Process
Plating is a process that uses chemical baths to apply a metal coating to an object’s surface. The chemical baths contain different metals in solution, which form a metal alloy when applied. An electrical current is then passed through the bath, allowing the metal alloy to adhere to the object’s surface. Plating can be used on objects made from many different materials, including plastic, wood, or metal. It is often used for decorative purposes as well as for protection against corrosion and humidity.
Electroplating Process
Electroplating is similar to plating in that it also applies a metal coating by using an electrical current passed through a chemical bath of metals in solution. However, unlike plating, electroplating only works with objects made of a conductive material such as copper or brass. The electrical current causes ions of the metal in the solution to bond with the object’s surface, forming a thin layer of metal over top of it. Electroplated objects are often more durable than those coated through plating since they can withstand higher temperatures and pressures without wearing away or corroding.
Difference Between Plating and Electroplating
- Plating is a process in which a metal is coated with another metal. This can be done for a variety of reasons, including to protect the metal from corrosion or to give it a different appearance.
- Electroplating is a type of plating in which an electric current is used to coat the metal with another metal. This process is often used to coat metals with a thin layer of another metal, such as gold or silver.
- Plating can be done using a variety of methods, including chemical plating and electroplating. Chemical plating involves using chemicals to coat the metal, while electroplating uses an electric current.
- Plating can be used for a variety of purposes, including protecting the metal from corrosion, giving it a different appearance, or increasing its conductivity.
- Electroplating is often used for decorative purposes, such as coating metals with a thin layer of gold or silver.
Conclusion:
When it comes to deciding between plating and electroplating for your project or product, there are several factors you should consider before making your decision. Plated objects tend to be more decorative while electroplated ones are more durable; however, electroplated objects must be made out of conductive materials in order for the process to work properly, while plated objects can be made from any material (although some may require additional treatments before being coated). Ultimately, you should base your decision on what type of results you want from your finished product—do you need something that looks nice but isn’t necessarily built to last or do you need something that will stand up against wear and tear? No matter which option you choose, both processes have their own advantages when it comes to creating beautiful products that last long into the future! Intended Audience: DIYers/craftspeople/manufacturers/artists looking for information about these two processes for their projects.

Pipingmart is a B2B portal that specializes in metal, industrial and piping items. Additionally, we share the latest information and information about materials, products and various types of grades to assist businesses that are involved in this business.