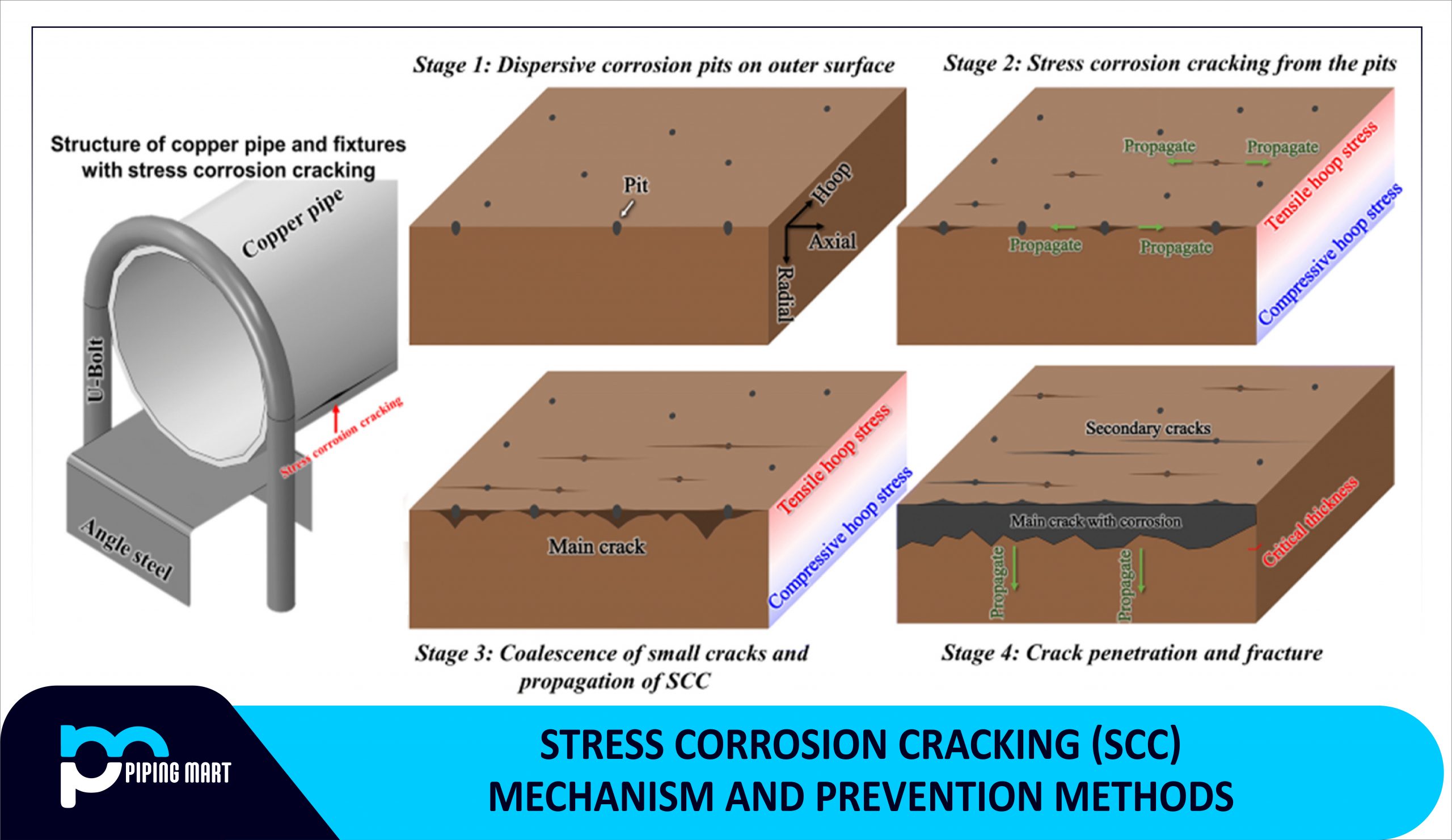
Stress Corrosion Cracking (SCC) is a gradual degradation process of engineered materials in corrosive environments. Every year, many ductile metals and alloys fail because of stress corrosion cracking, which begins with the initiation, propagation, and expansion of a crack to a destructive limit when exposed to a corrosive environment. Because stress corrosion cracking is alloy and environment-specific, the mechanism varies widely. A metal that exhibits SCC characteristics in one environment may not be susceptible to SCC attack in the other. However, the actual stress corrosion cracking process has not been thoroughly researched.
Stress Corrosion Cracking is a slow and gradual failure process. SCC can begin and spread with little or no indication of outside corrosion. Surface flaws produced by corrosion, wear, or other factors can result in the formation of cracks.
Stress corrosion cracking (SCC) is a kind of intergranular corrosion that occurs in the steel industry and results in crack initiation in a corrosive environment. Stress corrosion cracking is a major concern considering steel is the most commonly used industrial material for facilities such as piping, power plants, chemical industries, buildings, and so on.
Reasons behind Stress Corrosion Cracking
The failure of the stress corrosion cracking approach is caused by three major factors:
- Tensile strength (usually because of operational applied stress, thermal stress, or residual stresses from welding and fabrication)
- Corrosive Environment
- Susceptible material in a specific metallurgical state causes component failure prematurely.
Temperature and time are two more factors that might cause stress corrosion cracking.
The presence of cracks and other imperfections on the parts accelerates the deformation processes in SCC. SCC failures are rapid and catastrophic in nature, and they often occur at significantly lower stress levels than the yield stress. The following are some common instances of SCC:
- Seasonal cracking of brass in an ammonia-rich environment
- Stainless steel sensitization and stress corrosion cracking occur in the presence of caustic, chlorides, and polythionic acid.
Classification of Stress Corrosion Cracking
Various forms of stress corrosion cracking have been discovered, depending on the actual SCC process.
- Chloride Stress Corrosion cracking: It is widespread in austenitic stainless steels in the presence of chloride ions and oxygen when combined with tensile mechanical stress at high temperatures.
- Caustic Embrittlement: In a caustic environment, it is common in stainless steel with high hydrogen content.
- In the oil and chemical industries, SCC cracking of steel occurs in a hydrogen sulfide condition.
- Sessional Cracking: Brass cracking in ammonia environments.
- Craze Cracking: Cracking of polymeric materials as a result of applied stress and environmental reaction.
Stress Corrosion Cracking Features
SCC has the following unique Characteristics:
- Stress corrosion Cracking failure occurs at stress levels much lower than the material’s yield stress.
- Although SCC materials are ductile, their failure mechanism is brittle.
- Corrosion is the most common cause of cracks in stress corrosion cracking.
- Intergranular and transgranular cracks are the main characteristics of stress corrosion cracking at the microscopic scale. Intergranular cracks form at grain boundaries, whereas transgranular cracks form across grains.
Materials affected by Stress Corrosion Cracking
Stress Corrosion Cracking attacks are possible on the following materials:
- Stainless steels (temperatures ranging from 415°C to 850°C in chloride, caustic, and polythionic acid environments)
- Carbon Steel (in carbonates, caustic solutions, nitrates, phosphates, seawater solution, acidic H2S, and high-temperature water environments)
- Copper and copper alloys (in ammonia, amine, and water vapor-containing environment)
- Aluminum and aluminum alloys (in moist environments with NaCl solution)
- Titanium and titanium alloys (in the presence of seawater, fuming nitric acid, and methanol-HCl)
- Polymers (in acidic and alkaline environments)
- Ceramics
Stress Corrosion Cracking in Welding
The main cause of stress corrosion cracking is residual stress created during welding and manufacturing operations. The residual stress caused by welding is critical to the stress corrosion cracking of metal alloys. Welding stress corrosion cracking is induced by non-uniform temperature fluctuations during the welding process. Furthermore, the solid-state transformation of austenite to martensite during cooling creates high residual stresses when welding certain steel grades. Martensite is generated by quenching the austenite containing carbon atoms at a rapid cooling rate in carbon and low alloy steels. In this case, the carbon atoms are unable to diffuse out of the crystal structure and form cementite. This raises the volume of the metal, resulting in high residual stress.
Stress Corrosion Cracking Mechanisms
Various stress corrosion cracking mechanisms are common in the industry, depending on the type of material and environment. Some well-known SCC mechanisms include:
- Mechano-electrochemical model
According to this stress corrosion cracking process, there are pre-existing areas in an alloy microstructure that become sensitive to anodic dissolution.
- Film rupture model
The film rupture SCC mechanism is well-known for alloys with a passive layer on their surface. Corrosion begins after plastic deformation in this technique. The plastic pressure splits the film, exposing the underlying metal to the corrosive atmosphere. Soon after, a localized SCC attack begins in those parts, and the cycle continues, causing the cracking to develop.
- Adsorption phenomenon
Material embrittlement in the vicinity of a corroding area is considered by the SCC mechanism.
- Pre-existing Active Path Model
Intermetallics and compounds are formed in pre-existing paths such as grain boundaries, which are susceptible to SCC attack.
Stress corrosion cracking prevention measures
Because the process of stress corrosion cracking is not fully known, preventative techniques are based on actual experience. In general, one or more of the following measures can help to lower the risk of SCC:
- Because tensile stress is a primary factor in stress corrosion cracking, decreasing stress levels in parts reduces the possibility of SCC attack. Residual stress can be greatly reduced by applying an annealing treatment to the component.
- One technique for minimizing SCC attacks is to eliminate or reduce aggressive species in the environment where the component is placed. In the case of austenitic stainless steels, for example, keeping the chloride concentration below 10 ppm considerably minimizes the risk of SCC.
- Selecting
- Stress corrosion cracking-resistant materials will ensure protection from stress corrosion cracking.
- The use of cathodic protection helps in decreasing stress corrosion cracking.
- Phosphate and other organic and inorganic inhibitors can minimize the stress corrosion cracking effects in moderately corrosive environments.
- Applying a protective coating can be useful in some cases.
- Using shot-peening to create residual compressive stress on the component surface can help to minimize stress corrosion cracking.
- Lowering the temperature and electrochemical potential minimizes the risk of SCC.

Pipingmart is B2B portal specializes in industrial, metal and piping products. Also, share latest information and news related to products, materials and different types grades to help business dealing in this industry.