What Is Carburizing?
When carbon is diffused into a steel part’s surface layer during the case hardening process, it is known as carburizing. The grain structure of the steel is altered. The steel can now absorb carbon thanks to this alteration. Carburizing is perfect for creating sturdy, secure metals since it produces a wear-resistant coating.
Iron or steel is heated during the carburizing process, which involves the metal absorbing carbon while being heated in the presence of carbon-containing substances like charcoal or carbon monoxide. The goal is to harden the metal.
The amount of carbon in the affected region might change depending on the length of time and temperature. The depth of carbon diffusion usually increases with longer carburizing durations and higher temperatures.
When iron or steel is rapidly chilled by quenching, the core stays soft and durable due to a ferritic and pearlite microstructure. At the same time, the outer surface with a more effective carbon content hardens due to the change from austenite to martensite.
The following characteristics best describe this production process:
- It is given to low-carbon workpieces.
- The workpieces come into contact with a high-carbon gas, liquid, or solid.
- Workpiece cores retain most of their hardness and ductility, resulting in a hard workpiece surface.
- It can produce case hardness depths of up to 0.25 inch (6.4 mm).
- It can sometimes be used to correct unintended decarburization that occurred earlier in the production process.
The Method of Carburization
Steel is carbureted by applying heat to the metal’s surface while employing a carbon source. Low carbon steel’s surface hardness can be raised by carburization. Modern carburization methods utilize carbon-bearing gases or plasmas instead of directly applying charcoal packed around the sample to be treated.
The process is mainly dependent on the composition of the surrounding gas and furnace temperature, which must be carefully regulated since it may affect the microstructure of the remaining material. Carburization may be carried out in a vacuum chamber at extremely low pressures for applications where precise control over the gas composition is required.
Plasma carburization is used more often to enhance the surface properties of different metals, mainly stainless steel. The method is safe for the environment. It is also quite adaptable regarding component treatment since it offers equitable handling of components with complicated geometry.
Carbon atoms diffuse into the surface layers of metal during the carburization process. Since metals are composed of atoms that are securely linked together to create a metallic crystalline lattice, carbon atoms infiltrate into the crystal structure of the metal and either stay in solution or produce carbides when they interact with other elements in the host metal.
If the carbon doesn’t dissolve, the steel is heat-treated to make it harder. Both processes make the metal’s surface more robust; the first creates pearlite or martensite, and the second forms carbides. Both of these substances are tough and abrasion-resistant.
The temperature range for gas carburizing is typically between 900 and 950 °C.
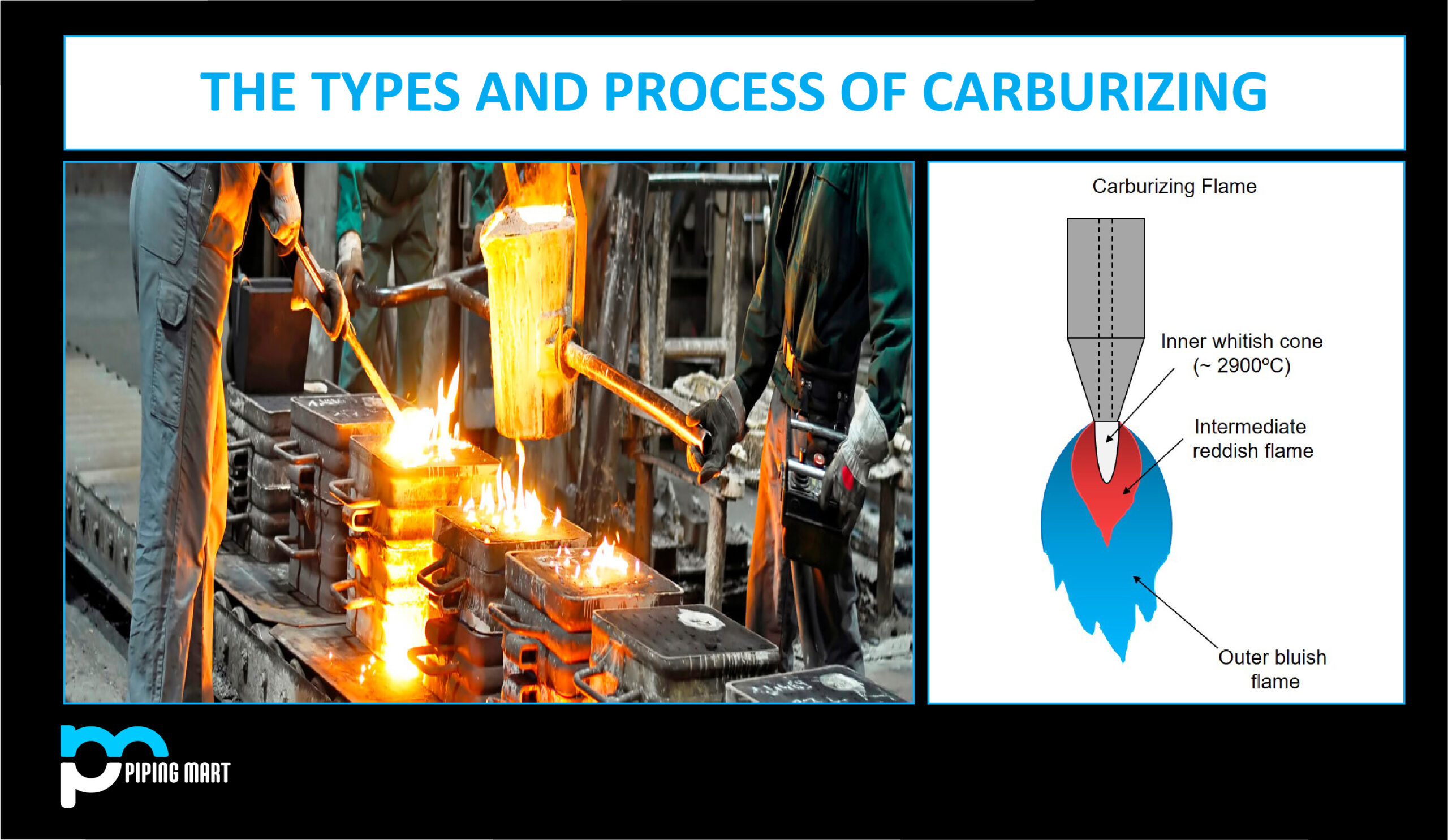
A carburizing flame in oxy-acetylene welding is one with less oxygen, which results in a sooty, lower-temperature flame. It is frequently used to anneal the metal to make the metal more pliable and flexible during the welding process.
Types Of Carburizing
Solid, liquid, and gas carburizing were the three different carburizing processes used in the past, depending on the carbon source. Following this, charcoal, molten salt, and carbon-containing gases like natural gas and propane are employed.
The basis for all three processes is the quenching-induced conversion of austenite to martensite. The increase in carbon content at the surface needs to be significant enough to produce a martensitic layer strong enough to withstand wear, approximately 700 HV.
Typically, upon diffusion, 0.8 to 1.0% C of carbon is needed on the surface. These procedures may be used on a range of carbon steels, alloy steels, and cast irons with a maximum mass carbon concentration of 0.4% and a specific mass carbon content of less than 0.25%. Oxidation or decarburization might result from improper heat treatment.
Carburizing may be utilized as a continuous process and is appropriate for large-volume surface hardening, even though it is a somewhat slow process.
Different forms of carburization are frequently employed:
- Gas Carburizing
When carbon steel is gas-carburized, it is heated to the austenitizing temperature while exposed to a carbon-rich environment. It is typical to combine hydrocarbon enrichment with a carrier gas, such as endothermic (“Endo”) gas (natural gas or propane).
The component is kept in an oven with a neutral carrier gas, often a combination of N2, CO, CO2, H2, and CH4, and an atmosphere of methane or propane. Methane (or propane) decomposes into atomic carbon and hydrogen on the component’s surface at the carburizing temperature, with the carbon diffusing into the surface.
Carburizing timeframes range from 2 hours for a housing with a depth of 1 mm to a maximum of 36 hours for a housing with a depth of 4 mm, with a typical temperature of 925 °C. Typically, oil is used as the quenching medium. However, it can also be water, salt, caustic soda, or polymer.
- Vacuum Carburizing
This carburizing procedure takes place in an oxygen-free, low-pressure atmosphere. Gaseous hydrocarbons, such as methane, are used in this process. The absence of oxygen in the surroundings means that the carburizing temperature may be raised without fear of oxidation. The carbon solubility and diffusion rate increase with temperature, reducing the time needed for case depth.
- Liquid Carburizing
Case hardening of steel or iron components is accomplished by liquid carburizing. The parts are kept above Ac1 in a molten solution that infuses the metal with either carbon and nitrogen or only carbon. Most liquid carburizing baths contain cyanide, which adds nitrogen and carbon to the case.
Carburization of liquids or cyanides is accomplished by submerging the component in a salt bath heated to 845°C – 955 °C. The salt is often a toxic combination of cyanide, chloride, and carbonate. A small quantity of nitrogen to the surface, increasing its hardness even further. The quickest carburizing method is only appropriate for small batch quantities.
- Solid Carburizing (Pack Carburizing)
In the solid or pack carburizing process, carbon monoxide from a solid compound breaks down into nascent carbon and carbon dioxide near the metal surface. Carburizing involves carbon steel, carbon steel with an aluminum coating, or heat-resistant alloys of iron, nickel, and chromium.
The parts are in a sealed box with a carburizing medium surrounding them. Typically, the medium is a mixture of barium carbonate and coke or charcoal. As the CO generated dissociates into CO2 and carbon, which seep through the surface of the components, the process is a process of gas carburization.
Temperatures typically range from 790 to 845 °C for 2 to 36 hours. Because packing carburizing is the least complex carburizing process, it is still an extensively utilized technique.
- Carbonitriding
Similar steel is used for carbonitriding, albeit the carbon concentration by mass can range from 0.4-0.5%. The technique is especially well suited for hardening the surface of parts that need a hardened core, including gears and shafts.
In a variation of gas carburizing called carbonitriding, ammonia—the nitrogen source—is added to methane or propane.

Pipingmart is B2B portal specializes in industrial, metal and piping products. Also, share latest information and news related to products, materials and different types grades to help business dealing in this industry.