What is Zirconium?
The unique physical and chemical characteristics of zirconium (Zr), a greyish-white metal, make it ideal for a variety of scientific and industrial applications. The mineral zircon in its silicate form and, less frequently, the oxide mineral baddeleyite are the two most prevalent forms of zirconium, the 20th most abundant element in the planet’s crust.
Zirconium metal and its alloys are used in a variety of products, including television screens, catalytic converters, vacuum tubes used in electronics, surgical equipment, and implants. Zirconium is also an essential component of steel and aluminum alloys. Zirconium compounds also make suitable surface coatings in the paper and packaging industries because of their superior water resistance and durability.
Zirconium silicate (ZrSiO4), also known as zircon, is a naturally occurring mineral that is extracted from prehistoric mineral sand deposits and used to make the majority of the zirconium compounds and zirconium metal produced globally.
After mining and the creation of a heavy mineral concentrate, zircon is separated, beneficiated, and commercialized as zircon sand. This sand is used either directly in specific applications (foundry sands, including investment casting), or it is processed for use in refractories, ceramic opacification, or a variety of zirconium compounds from which the metal can be produced.
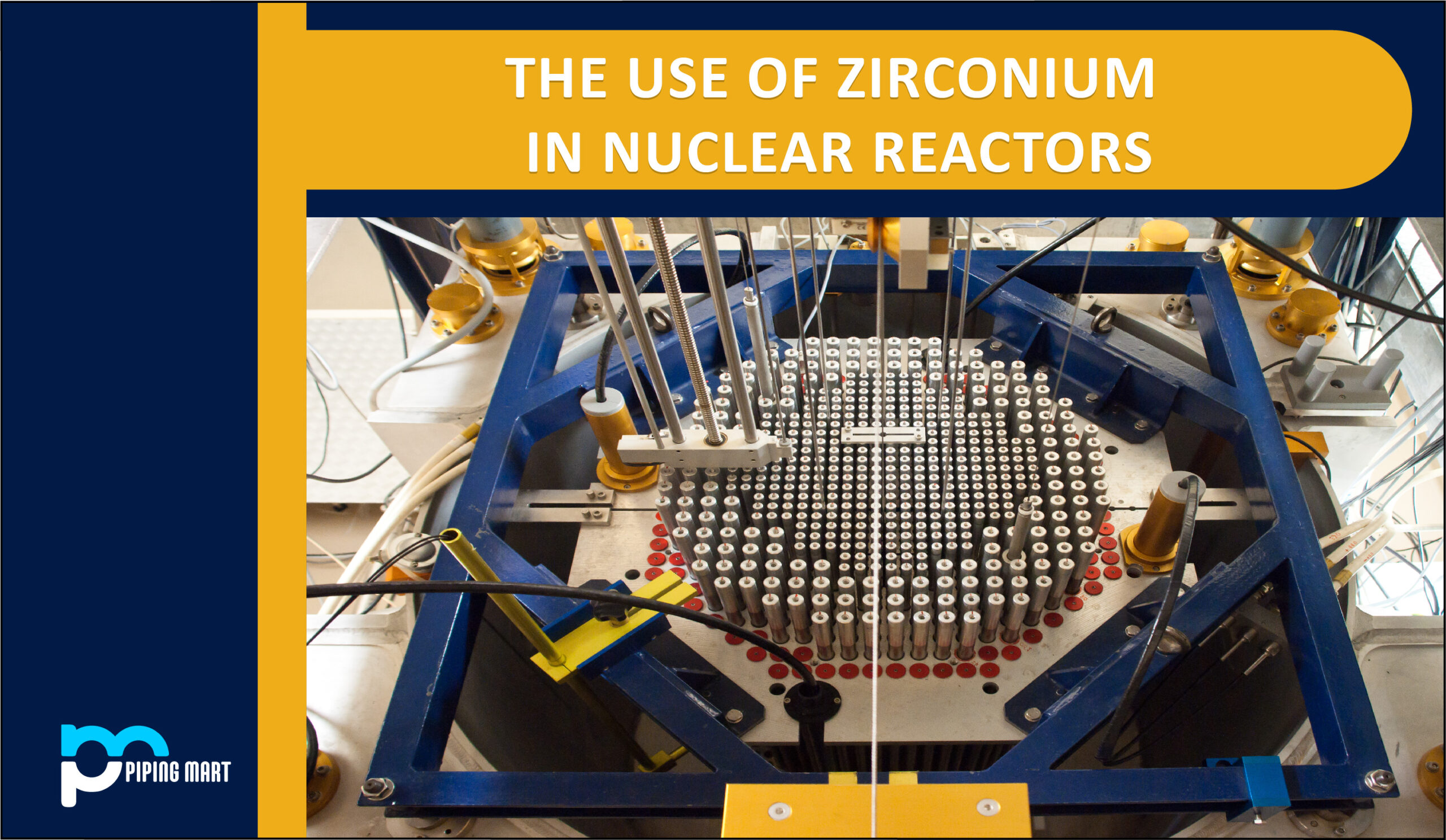
Uses of Zirconium in Nuclear Reactors
Zirconium is a rare metal with exceptional strength and a high melting point among many other appealing qualities. It is extensively employed in the sectors of atomic energy, nuclear reaction, aerospace, and defense. The nuclear power sector was largely responsible for the early development of zirconium metallurgy, and zirconium alloys are today recognized as the tested structural material for nuclear fuel cladding in light water reactors. This is primarily due to the alloy’s exceptional combination of high corrosion resistance in water at 300 C and low thermal neutron capture cross-section.
The cladding, or outside coating, for the cylindrical fuel rods that fuel nuclear reactions is made of zirconium in nuclear reactors. Uranium oxide or other fissionable material pellets are enclosed within the zirconium cladding.
Zirconium is the ideal metal for this use since it only absorbs a small portion of the neutrons created during a fission reaction and has a strong heat and chemical corrosion resistance. A nuclear fuel cladding material that could endure corrosion at high temperatures and for extended periods, preserve its integrity in an environment of intense radiation, and not absorb the neutrons necessary for the nuclear reaction was needed for a pressurized water reactor (PWR).
Any structural material used in a nuclear reactor must have low neutron absorption because massive amounts of reaction-generated neutrons must be allowed to interact simultaneously with all the nuclear fuel contained inside dozens or hundreds of fuel rods. The essential chain reaction is maintained by this interaction in the reactor’s core.
The use of zirconium metal in nuclear reactors has many goals and one such is to either slow down or speed up the fission process. Atoms are divided during fission, which produces heat and makes it possible to make electricity. Zircon is carbo-chlorinated or even plasma-dissociated at high temperatures to recover zirconium metal from zirconium ore. Zircon must be separated from hafnium, which is a costly technological procedure and a technically difficult process given their similarities.
In addition to being extremely ductile and hard, zirconium also possesses a low thermal electron absorption cross-section. The harder it is for a zirconium alloy to corrode, the purer it is. Zirconium is employed in structural applications such as reactor core materials, positioning frameworks, plugs, moderators, pressure tubes, and support grids. This is why zirconium metal, which is used in nuclear reactors as zirconium alloy, has seen a surge in demand along with the growing need for nuclear energy. Zirconium has established itself as a dependable metal although other alternatives like ceramics have frequently been investigated.
Nuclear-grade zirconium alloys typically consist of more than 95% zirconium and less than 2% of additional metals, including tin, niobium, iron, chromium, nickel, and others, which are added to enhance mechanical qualities and corrosion resistance. Zircaloy 4 has been the alloy most frequently used in PWRs up to this point, although new zirconium-niobium-based alloys that demonstrate superior corrosion resistance are currently replacing it. The highest temperature that zirconium alloys can be utilized in water-cooled reactors depends on how resistant they are to corrosion. The most popular zirconium alloys, Zircaloy-2 and Zircaloy-4, include iron, chromium, nickel, and the strong stabilizers tin and oxygen.
Tin serves as the primary alloying element in zircalloy alloys, which improves mechanical qualities. These alloys are widely used throughout the world. In this instance, however, the requirement for extra alloying was caused by the decrease in corrosion resistance in water and steam. Niobium added in addition likely uses a different technique to improve things. The ability of niobium alloyed metals to passivate with the production of protective coatings accounts for their high corrosion resistance in water and steam at temperatures of 400–550°C.
Both commercial nuclear electric generating plants and military reactors use zirconium cladding, which is typically an alloy of zirconium, tin, iron, nickel, and chromium. The sale of zirconium cladding does not necessarily indicate that the user intends to construct military reactors that can produce bomb fuel.
Zirconium silicate complexes, from which the metal is recovered, are mined, among other places, in the former Soviet Union, Australia, Brazil, and Florida. The zircon gemstones contain metal.
Aluminum, beryllium, and stainless steel were compared and found to be inappropriate. Because zirconium contains roughly 2% (by weight) of hafnium (Hf), which affects its high degree of neutron absorption cross-section, first testing with zirconium revealed that it absorbed neutrons required for the fission process.
Separation of zirconium and hafnium
For nuclear energy uses, zirconium and hafnium must be separated, although it is quite difficult, some places have managed to accomplish this. As a result, zirconium in its purest form absorbed very few neutrons.
In this liquid-liquid extraction procedure, hydrochloric acid is used to dissolve the combined ZrHf-chloride. In a counter-current liquid-liquid extraction technique, the Zr and Hf ions are complexed with ammonium-thio-cyanate before Hf is extracted with MIBK. Zr-containing aqueous phase is combined with sulfuric acid and ammonium hydroxide to precipitate Zr as hydroxide.
After filtration, the Hf is removed from the MIBK with hydrochloric acid, and the Zr-hydroxide is calcined to ZrO2. To get a ZrCl4 that is Hf-free and can then be reduced to pure zirconium, the carbo-chlorination procedure must be repeated.
Benefits of Zirconium
Biocompatibility: Zirconium is considered to be highly biocompatible.
Durability: Zirconium is formed of crystal, which makes it almost indestructible and able to survive even the most ferocious gnawing and biting. Zirconium is five times more powerful than porcelain. Zirconium products are made with a milling process that makes them almost unbreakable. Moreover, they possess the ability to withstand extreme cold and hot conditions.
Long-lasting: Zirconium products are more durable and also last longer. This is mostly because zirconium is less likely to chip or crack. They may be easily molded, colored, and adjusted.
Cost of Zirconium
These alloys are frequently used in heat exchangers and piping systems for the chemical processing and nuclear sectors due to their low cost. Zirconium is a by-product of tin mining as well as the extraction and processing of titanium minerals. While zircon prices grew gradually from 360 USD to 840 USD per tonne from 2003 to 2007, the cost of unwrought zirconium metal dropped from 39,900 USD to 22,700 USD per tonne. Because the reduction operations are expensive, zirconium metal is substantially more expensive than zircon. All prices differ greatly depending on the purity.
Production of Zirconium
Due to the unique chemical characteristics of zirconium, special processes are needed to produce zirconium metal. The Kroll process, which reduces zirconium chloride with magnesium metal, produces the majority of Zr metal from zircon (ZrSiO4). The reduction of zirconium chloride to metallic zirconium by magnesium is the main component of the Kroll process. Hafnium, whose neutron absorption cross-section is 600 times that of zirconium, is generally found in concentrations of 1-5% in commercial non-nuclear grade zirconium. For reactor uses, hafnium must be almost completely eliminated (down to 0.02% of the alloy).
Oxidation of Zirconium Alloys
One of the nuclear industry’s most thoroughly researched processes is the oxidation of zirconium alloys. When zirconium reacts oxidatively with water, hydrogen gas is released. Part of this gas diffuses into the alloy and creates zirconium hydrides. Because the hydrides are less dense and structurally weaker than the alloy, when they form, the cladding blisters and cracks, a condition known as hydrogen embrittlement. There are a lot of publications dealing with the oxidation of zirconium alloys at moderate temperatures of around 800 K and below, even if many of them are written to address the reaction of fuel and steam with zirconium alloys in the event of a nuclear disaster.
Zr + 2H2O→ZrO2 + 2H2
Zr-base alloys exothermically react with steam at high temperatures, which makes the reaction much more intense and dangerous for nuclear power plant safety during mishaps like a coolant loss accident (LOCA). High-temperature oxidation is primarily problematic because zirconium cladding interacts quickly with water vapor. For several Zr-based alloys, the oxidation kinetics appears to be parabolic in the temperature range of 1000–1500 °C.
What is Zircaloy – Zirconium Alloy – 4
In contrast to Zircaloy-2, which contains nickel, Zircaloy-4 (UNS R60804) has a higher, more tightly controlled iron content. Pure zirconium is mechanically comparable, stronger, less ductile, and has high corrosion resistance. This alloy is also utilized in nuclear applications because, when subjected to corrosion in water and steam, it absorbs less hydrogen than Zircaloy-2.
The main goal of developing Zircaloy-4 from Zircaloy-2 was to lessen its propensity to pick up hydrogen. The same composition requirements, therefore, apply, except for nickel, which is restricted to a maximum of 0.007%, and iron, whose range is decreased to 0.18%.
Hydrogen Embrittlement of Zirconium Alloys
The use of cladding stops radioactive fission products from the fuel matrix from escaping and contaminating the reactor coolant. Numerous fuel failure root causes have been discovered in the past. These causes were primarily fabrication flaws or fretting in the early stages of PWR and BWR operations. Another of the causes might be:
Hydriding internally- Inadvertently putting hydrogen-containing materials into a fuel rod can cause hydriding, which makes the fuel coating more brittle. The main sources of hydrogen were organic contaminants or residual moisture in fuel pellets or rods. Improvements in manufacturing have virtually eliminated this cause of failure.
Hydride cracking that is delayed (DHC)- Delayed hydride cracking is the crack initiation and propagation of hydrides that can fracture before the crack tip. Long cracks that start at the cladding’s outer surface and spread in an axial or radial direction might cause this kind of failure. High burnup operation might be restricted by this failure mechanism.
Properties of Zirconium Alloy
Material characteristics are intense qualities, which means they are independent of the mass present and can shift at any time from one position in the system to another. Studying its structure and connecting it to its properties forms the foundation of materials science (mechanical, electrical, etc.). Once a zirconium user is aware of this structure-property connection, they can explore the relative performance of the material in a particular application. The primary determinants of its structure and, consequently, its attributes are its chemical constituents and how they were mixed to produce its final form.
- The density of Zirconium: A typical zirconium alloy has a density of 6.6 g/cm3 (0.24 lb/in3). Its intense attribute is defined as mass divided by volume.
Since a substance’s density is calculated by dividing its total mass by the total volume that it occupies, it follows that zirconium’s density is highly influenced by both its atomic mass and atomic number density.
Atomic mass: The atomic nucleus of zirconium in reactors, which only takes up around 10 to 12 percent of the overall volume of the atom or less, is responsible for carrying the atomic mass. It also carries all the positive charge and at least 99.95 percent of the atom’s total mass. Therefore, the mass number governs it (number of protons and neutrons).
Atomic Number Density: The number of atoms of a certain kind per unit volume (V; cm3) of the substance is known as the atomic number density (N; atoms/cm3), which is related to atomic radii.
Crystalline Form: The crystal structure of crystalline zirconium has a considerable impact on its density. The FCC structure has the highest packing factor (74%). Austenite, aluminum, copper, lead, silver, gold, nickel, platinum, and thorium are some of the metals that possess FCC structures.
- Mechanical Properties: Zirconium has an acceptable combination of mechanical properties, which is why it is usually chosen for usage in a variety of applications including a nuclear reactor. Material qualities are essential for structural applications and must be taken into consideration.
- Strength of Zirconium Alloy: The strength refers to its capacity to sustain an applied load without breaking or deforming plastically. It is a very crucial property to consider in a lot of applications. The main factor that goes into determining a zirconium’s strength is the relationship between the external loads that are placed on it and the subsequent deformation or alteration in its dimensions. The strength of zirconium is determined by its capacity to bear this applied load without breaking down or deforming plastically.
- Ultimate Tensile Strength: On a stress-strain curve, the ultimate tensile strength is at its highest. This is equivalent to the maximum stress that a structure in tension can withstand. The fracture will occur if this stress is applied and kept in place. This value frequently exceeds the yield stress by a large margin (as much as 50 to 60 percent more than the yield for some types of metals). About 514 MPa is the maximum tensile strength of zirconium. The ductile material experiences necking, which is a localized reduction in cross-sectional area, when it reaches its maximum strength. The ultimate strength is the highest stress on the stress-strain curve. Although deformations may continue to grow, once the maximum strength has been reached, the stress typically diminishes. Because it is an intense property, the size of the test specimen has no bearing on its value. However, it also depends on other elements including how the specimen was prepared, whether or not there are surface flaws, and the temperatures of the test environment and the material. Aluminum has an ultimate tensile strength of 50 MPa, while exceptionally high-strength steel can reach 3000 MPa.
- Yield Strength: The yield point on a stress-strain curve is the location where elastic activity ends and plastic behavior starts. While yield point is the starting point of nonlinear (elastic + plastic) deformation, yield strength is the attribute defined as the stress at which it begins to deform plastically. The material will elastically bend before reaching the yield point and will resume its original shape once the applied tension is removed. Some portion of the deformation will become permanent and irreversible once the yield threshold is passed. About 381 MPa is the yield strength of the zirconium. The yield point phenomenon is a behavior that some steels and other materials displayed. Yield strengths range from greater than 1400 MPa for very high-strength steels to less than 35 MPa for low-strength aluminum.
- Young’s Modulus of Elasticity: It is typically measured by tensile testing and represents the elastic modulus for tensile and compressive stress in the linear elasticity regime of uniaxial deformation. Zirconium has Young’s modulus of elasticity of around 99 GPa. A body will be able to regain its proportions after the load is removed, up to limiting stress. The atoms in a crystal move out of their equilibrium position as a result of the applied stresses. With the same amount of displacement, all the atoms maintain their relative geometry. All the atoms return to their original locations after the tensions are released, therefore there is no lasting deformation. In the elastic zone, the stress is inversely proportional to the strain, and the slope is determined by Young’s modulus. The longitudinal stress divided by the strain equals Young’s modulus.
- Thermal Characteristics of Zirconium Alloy: The thermal characteristics describe how they react to variations in temperature and the application of heat. A solid’s temperature and size grow as a result of absorbing energy in the form of heat. However, the way various materials respond to heat application varies. In the actual use of solids, heat capacity, thermal expansion, and thermal conductivity are frequently crucial characteristics.
- Melting Point: Zirconium, has a melting point of roughly 1850°C. A substance’s transition from the solid to the liquid phase is known as melting. The temperature at which this phase change takes place is known as a substance’s melting point. The melting point specifies a circumstance in which an equilibrium between the solid and liquid is possible.
All these properties have proven to be extremely crucial and helpful in a variety of applications, especially in nuclear reactors.

Pipingmart is B2B portal specializes in industrial, metal and piping products. Also, share latest information and news related to products, materials and different types grades to help business dealing in this industry.