What is an ORP?
The term “ORP” refers to “oxidation-reduction potential,” which is a measurement of a chemical substance’s propensity to oxidize OR reduce another chemical substance in millivolts. An ORP meter’s positive reading denotes the presence of an oxidizing agent, whereas a negative reading denotes the presence of a reducing agent.
When an object doesn’t have enough electrons, it will aggressively seek them out by oxidizing another substance, which is a chemical reaction (i.e., loses electrons to another agent). Substances having positive ORP values are oxidizing agents because they are trying to pick up electrons.
Conversely, electrons with an excess of ions can tolerate losing ions to oxidizing agents without experiencing self-destabilization. They are referred to as reducing agents or antioxidizing agents for this reason. A chemical is more oxidizing or antioxidizing depending on whether its ORP measurement is higher or lower (positive or negative).
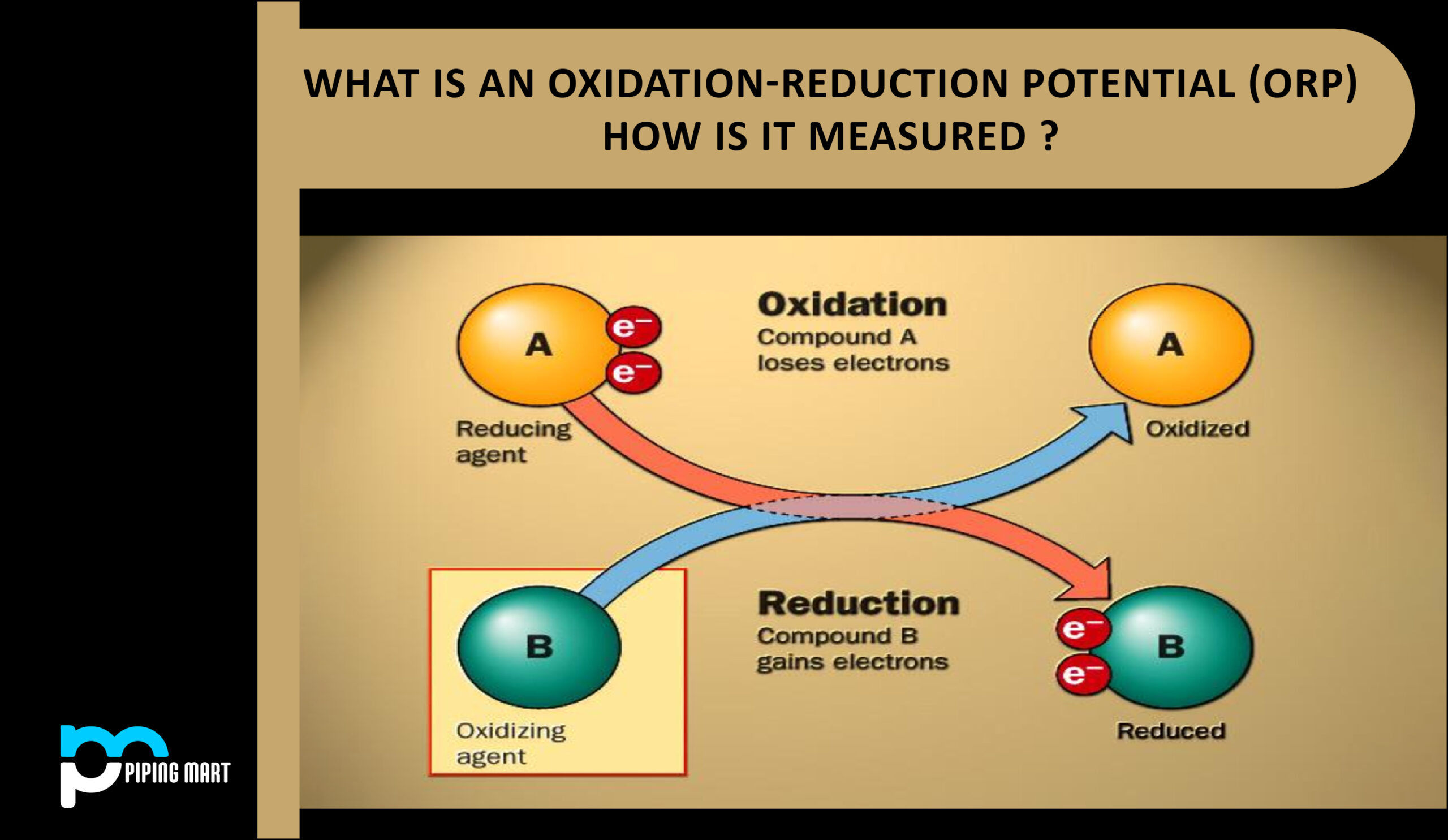
Oxidation: An atom, molecule, or ion undergoes oxidation when it loses electrons. The word comes from the fact that it may or may not be followed by the addition of oxygen. Iron rusting and wood burning are two common examples.
A substance’s oxidation state increases after it has been oxidized. Various oxidation states are possible for a wide variety of compounds. Sulfur is a good example because it can exist in the oxidation states of -2 (H2S), 0 (S), +4 (SO2), and +6 (S) (SO4 -2). Multiple oxidation states in a substance allow for progressive oxidation from one higher oxidation state to the next.
Redox couples are oxidation states of a chemical that are close to one another. The redox pair in the example below is Fe+2/Fe: Fe= (Fe+2) + 2e-
Because the electrons lost by the iron atom cannot exist in solution and must be taken up by another material in solution, the chemical equation for oxidation described above is known as the half-reaction. For iron to be completely oxidized, another material that will be reduced must also be present. Thus, just half of the entire reaction is represented by the oxidation reaction for iron.
Reduction: The net gain of electrons by an atom, molecule, or ion is referred to as reduction. A chemical substance’s oxidation state is decreased when it is reduced. Substances that can display several oxidation states can also be successively reduced from one oxidation state to the next lower oxidation state, just as was the case with oxidation.
The half-reaction for the reduction of chlorine is represented by the chemical equation below: In the example above, the redox pair is Cl2/Cl- (chlorine/chloride). Cl2 + 2e- = 2Cl-
Reduction processes always follow oxidation reactions. The electrons obtained in reduction reactions must originate from a source, whereas the electrons lost in oxidation must find another substance to go to.
The electrons lost in the oxidation process and the electrons obtained in the reduction reaction must balance out when two half-reactions are joined to form the total reaction.
Reduction = Cl2 + 2e- = 2Cl- Oxidation = Fe = (Fe+2) + 2e-
Reaction overall: Fe + Cl2 = FeCl2.
Iron (Fe), a reductant or reducing agent, reduces chlorine (Cl2) in the reaction described above.
On the other hand, chlorine (Cl2), which is referred to as an oxidant or oxidizing agent, oxidizes iron (Fe)
Why is ORP Used?
Since it is practical, typically accurate, and enables us to electrically monitor what is happening in the water, ORP is extensively utilized. ORP is a real-time reading of chlorine’s performance even though it does not monitor the amount of chlorine. The majority of operators concur that from a health standpoint, disinfection is what matters; chlorine content may not always be as significant as its efficacy. To get more done with less, the ideal situation would be to have a tiny bit of chlorine that is incredibly effective.
Anything above 750 mV is considered good; anything over 800 mV is considered great for ORP. Other than that, the precise figures depend on operator judgment, but it is typically agreed that higher is better.
Measurement of ORP
The measurement electrode and a reference electrode make up the cell. The test solution’s ORP is determined by the voltage between the electrodes. Despite being displayed separately, the electrodes are typically paired with ORP sensors, electrodes, and a temperature element to form a single sensor in most operations.
The measurement’s temperature must be supplied because ORP is temperature-dependent.
ORP Electrode: The use of an inert metal electrode (platinum, occasionally gold), whose low resistance allows it to give up electrons to an oxidant or take them from a reductant, is the basis for the ORP measurement. Due to the buildup of charge equal to the ORP of the solution, the ORP electrode will continue to absorb or give up electrons until it generates a potential. An ORP measurement’s typical accuracy is 5 mV.
A low rate of electron exchange can occasionally make it difficult for certain chemical compounds to exchange electrons with the ORP electrode (exchange current density). In these circumstances, a second redox pair in the solution may cause ORP to react more strongly (like dissolved oxygen). It is advised that new ORP applications be tested in the lab before going live online because this causes measurement mistakes.
Reference Electrode: The same silver-silver chloride electrode that is used to measure pH is often utilized as the reference electrode for ORP measurements. Since the mV changes detected in the majority of ORP applications are substantial, as will be demonstrated, some offset in the reference is acceptable in ORP measurements as opposed to pH measurements. An ORP sensor may employ a silver billet or even a pH electrode as a reference in specialized applications (for instance, bleach manufacture).
Temperature Effects on ORP
Measurements of ORP are impacted by temperature in 2 different ways:
For a particular ratio of ionic activity, electrodes will have a varied output potential at various temperatures.
The effects of temperature on dissociation, activity coefficients, and interactions between ions in solution have an impact on ionic activity.
However, in most cases, ORP measurements do not account for temperature impacts because:
-There is no “standard” isopoint for all ORP reactions; instead, the isopotential point (the point of thermal independence) of ORP systems is unique to that specific redox reaction.
-The chemistry of the redox reaction can be rather complicated, especially if numerous ionic species that contribute to the reaction’s oxidation-reduction potential include different numbers of transferred electrons (and hence different values for n within one equation).
-At constant temperatures, as in process measurement and control, most ORP measurements are conducted.
ORP in Solutions
The standard potential for a half-reaction is based on the presumption that all of the chemical compounds depicted in the half-reaction have concentrations of 1 molar. However, the concentrations in a process can change apart from one another. Therefore, the ORP for each case must be calculated using the Nernst equation to determine the ORP of a specific solution.
Standard Potential
The standard potential of a redox couple, denoted by E°, tells us how quickly a chemical can be reduced or oxidized. Quite a few redox couples’ standard potentials and half-reactions are listed in reference books for each redox couple. All references are to the hydrogen ion/hydrogen (H+/H2) redox pair, which has a standard potential of 0 millivolts. Half response, represented as a drop in the standard potential, is mentioned. The oxidation half-standard reaction’s potential is given by the negative of the tabulated standard potential.
Importance of NERNST Equation for ORP
Observing the hypochlorous acid/chloride equation reveals several crucial ORP characteristics:
- The ORP is influenced by the amounts of each drug in the half-reaction (except water). As a result, the ORP of hypochlorous acid depends just as much on the pH (H+) and chloride ion (Cl-) as it does on the acid itself.
- The logarithm of the concentration ratio is a function of the ORP.
- This logarithm of concentration is multiplied by a coefficient equal to -59.16 mV divided by the number of electrons in the half-reaction (n). Since n = 2 in this instance, the coefficient is -29.58. Only the ORP will vary by 29.58mV for a shift in Cl-, HOCl, and H+ concentrations of 10 times.
- No particular temperature relationship is evident. Unlike pH, where a general ORP temperature behavior can be described, temperature can influence an ORP reaction in several different ways. As a result, temperature correction is nearly never applied to ORP readings.
The Nernst equation can be divided into individual logarithms for each ingredient, and the contribution of that substance over its expected concentration range can be determined to examine the impact of a specific substance on the half-reaction.
Applications of ORP
Although there are fewer applications for ORP measurement including bleaching procedures, production of bleach, cyanide waste oxidation, and a decrease in chromate waste.
Many people have found applying ORP to be confusing and frequently infuriating due to its dependence on the quantities of many chemical compounds. Knowing the half-reaction involved and the range of concentrations for all the compounds present in the half-reaction is essential when considering ORP for a specific application. To understand the anticipated ORP behavior, the Nernst equation must also be used.
To monitor and manage oxidation-reduction reactions, ORP is used in the destruction of cyanide, the dechlorination of chlorine and chlorine dioxide, the creation of hypochlorite bleach, and the monitoring of chlorine and chlorine dioxide scrubbers using bisulfite.
It is difficult to measure concentrations with ORP, but in some circumstances, it can be used to find leaks and determine whether an oxidant or reductant is present.
Last but not least, ORP is occasionally evaluated to regulate biological growth. The idea behind these applications is that microorganisms can be effectively eliminated at a minimum ORP value. This method has been applied to the chlorination of cooling towers and swimming pools. It should be noted that pH regulation is a component of each of these applications.
Testing the water quality in chlorinated swimming pools is arguably the most widespread application. An ORP measurement is said to be more insightful than a pH reading alone, which solely distinguishes acids (hydrogen ions) and bases since it takes into account all of the agents that are present in the sample (hydroxide ions). While backyard pools without built-in monitors may need testing with a portable ORP monitor, larger pools frequently contain inline ORP sensors.
By raising the water’s ORP level, active chlorine electrons neutralize pollutants. A safe ORP reading for chlorinated pools and spas is typically between 650 millivolts (mV) and 750 mV. The water’s overall ORP value gradually drops as time goes on and chlorine degrades, losing its oxidation potential.
Although the ratio of sanitizer in the water and ORP levels often correlate, ORP evaluates the net oxidation and reduction characteristics of all agents in the environment.
ORP meters are commonly used in the food processing and water treatment sectors to verify that water is free of contaminants and acceptable for recycling or consumption in addition to being used to check water quality in chlorinated pools. A positive ORP value will be present in bottled water or clean tap water.
Adjusting ORP Levels
To increase the water’s ability to fight oxidation, additional active chlorine can typically be added to swimming pools to control ORP levels. Total dissolved solids (TDS) in water may need to be balanced more intricately in more advanced water purification systems to get a better oxidation-reduction potential.
Investigate the particular features of the ORP meter an individual has chosen to learn more about how to use it and how to interpret ORP data.
Factors that affect ORP
The ORP may change as a result of several water chemical issues. The following are a few of the most typical swimming pools:
-pH Level: The ratio of hypochlorous acid (HOCl) to hypochlorite ion (OCl-) is higher and the ORP is higher the lower the pH. Here is a sophisticated graph that displays the ratio of strong chlorine, or HOCl, to oxygen (weak chlorine). Recent research demonstrates that this equilibrium spectrum only holds for non-stabilized water. Alternatively, water is devoid of cyanuric acid.
-Cyanuric Acid: The ORP is said to decrease when cyanuric acid concentrations rise (also known as chlorine stabilizer or conditioner), according to the US Centers for Disease Control (CDC). This is the fundamental justification behind the CDC’s new cap on CYA levels following a fecal occurrence. The new cap is about 15 ppm CYA.
It is necessary to fully comprehend and value the importance of sanitizers. The first line of defense against dangerous bacteria and other organisms in the water is residual sanitizers like chlorine. In general opinion, it is advised to keep the stabilizer levels as low as feasible because CYA weakens chlorine.
-Phosphates: They can cause a decrease in ORP indirectly.
Cyanide Treatment in the Metal Industry:
The destruction of the cyanide with chlorine is one of the most efficient ways to handle these waste materials. The following steps are involved:
-Utilizing pH and ORP control, cyanide is oxidized to cyanate.
-Nitrogen and carbon dioxide, two inert gases that can be safely released into the environment, are produced when cyanate is oxidized. pH and ORP control are also used at this stage.
Cyanide to Cyanate:
The pH of the waste is detected in the first reaction tank, and caustic (NaOH at 50% strength) is immediately introduced to automatically elevate the pH to 10 or higher.
After measuring the waste’s oxidation-reduction potential (ORP), chlorine gas (Cl2) is automatically administered to elevate it to 400 mV or higher.
The following reaction happens, and it takes between 5 and 10 minutes:
NaCNO + 2NaCl + H2O NaCN + Cl2 + 2NaOH NaCNO
Cyanate breakdown into Nitrogen and Carbon Dioxide
The pH of the waste is measured in the second reaction tank, and acid is injected to lower the pH to 7-8.
This process takes two to five minutes. When the third reaction tank detects the waste’s ORP, chlorine gas (Cl2) is immediately injected to raise the ORP to 600 mV or higher (While doing so, the pH controller keeps the set-point at 7-8 while adjusting for any acidity that may have been brought on by the addition of the chlorine gas).
The subsequent reaction takes place, and it takes 10 to 15 minutes:
6NaCl + 2CO2 + N2 + 2H2O = 2NaCNO, 3Cl2, and 4NaOH
The aforementioned reaction finally transforms the cyanide into innocuous substances, allowing the waste to be released.
Issues related to Concentration Measurement with OPR
Without a complete grasp of all the variables, ORP is frequently applied to a concentration measurement (such as the amount of chlorine in water). The issues with a concentration measurement can be listed when the equation for a hypochlorous solution’s ORP is taken into account :
- The ORP is dependent on pH (H+), chloride ion (Cl-), and hypochlorous acid in equal measure (chlorine in water). The ORP will be impacted by any modifications to the pH or chloride content. Chloride ion and pH must therefore be measured with great precision or carefully controlled to constant values to quantify chlorine reliably.
- Using the recorded millivolts as a starting point, the hypochlorous concentration can be calculated using the exponent of 10. An ORP measurement’s typical accuracy is 5 mV. The calculated concentration of hypochlorous acid will be wrong by more than 30% due to this inaccuracy alone. This inaccuracy will only increase if there is any drift in either the reference electrode or the ORP analyzer.
- Because the ORP is not corrected for temperature changes, the inaccuracy in the derived concentration is further increased.
In general, applying ORP to concentration measurements is not a smart idea. The bulk of ORP half-reactions is dependent on pH and almost always involves multiple substances. Any inaccuracies in the millivolt measurements are multiplied by the ORP’s logarithmic dependence on concentration.
Conclusion
The major indicator of a solution’s oxidizing or reducing strength is its oxidation-reduction potential. Every oxidation or reduction has a half-reaction that identifies all of the chemical components involved in the reaction. The logarithm of the substance concentrations involved in the half-reaction determines the ORP of the solution. The Nernst equation can be used to determine the ORP. Due to its logarithmic dependence on concentration and its dependence on numerous solution components, ORP is a poor method for determining concentration. The monitoring and management of oxidation-reduction reactions is the best application of an ORP meter.

Pipingmart is B2B portal specializes in industrial, metal and piping products. Also, share latest information and news related to products, materials and different types grades to help business dealing in this industry.