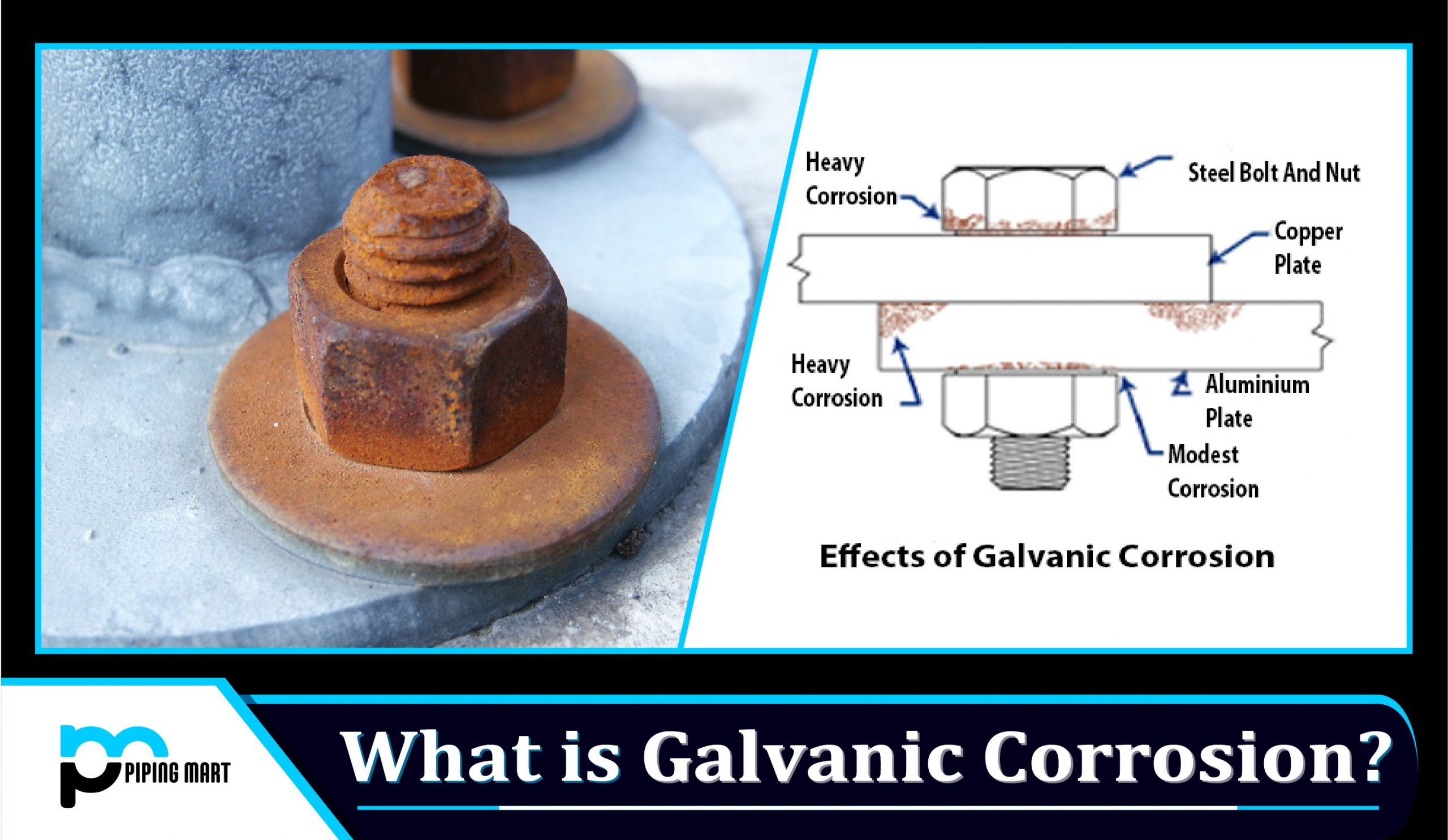
Galvanic corrosion is also commonly called bimetallic corrosion. It is an electrochemical process in which one metal corrodes before another metal is in contact with through an electrolyte.
When two metals that are not similar, are immersed in a conductive solution and electrically connected, galvanic corrosion occurs. The cathode, which is one metal, is protected, and at the same time, the anode is corroded. The rate of attacking the anode is accelerated with time.
To take an example, if two materials say aluminum and carbon steel are put in a conductive solution and electricity is passed, aluminum will corrode quicker than carbon steel. Carbon steel will receive protection.
The four elements that are necessary for corrosion to occur in a galvanic cell are:
- Anode: it is the negative electrode from where negative ions are discharged and positive ions are formed. Corrosion takes place at this electrode.
- Cathode: it is the positive electrode. Positive ions are discharged from this electrode and negative ions are formed.
- Electrolyte: it is the conductor which carries the current.
- Return current path: it is the metallic pathway that connects the cathode and the anode.
Ways to prevent galvanic corrosion:
- Materials with similar corrosion potentials should be selected.
- The electrical connection can be broken if the two metals are insulated from each other.
- The protective coating should be applied to both materials. The coating on the material on the cathode should be in a good condition, or else the galvanic corrosion could get worse.
- The two materials should be separated by inserting a suitably sized spacer.
- A sacrificial anode should be installed, which is anodic to both the metals.
- A corrosion inhibitor can be added to the environment.
If these methods are not practically possible, the rate of galvanic corrosion can be reduced by keeping the anode and cathode area large. Another way is that the design of the anode is such that the appropriate corrosion is allowed.

Pipingmart is B2B portal specializes in industrial, metal and piping products. Also, share latest information and news related to products, materials and different types grades to help business dealing in this industry.