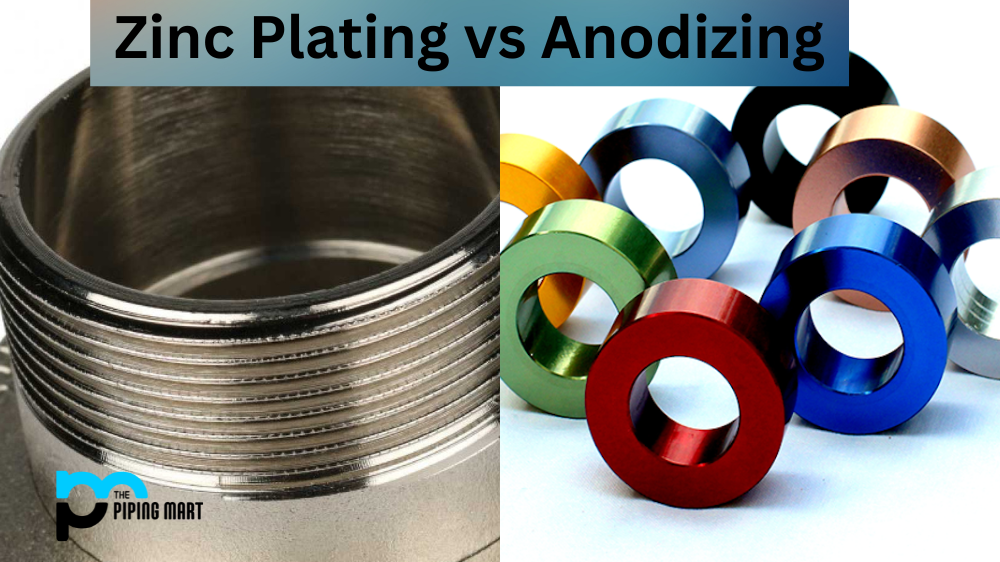
If you’re in the market to protect metal surfaces, you may be considering zinc plating or anodizing. Both of these processes are used to protect the metal from corrosion, but they do so in different ways. This blog post will explore the differences between zinc plating and anodizing and how they can help your products last longer.
How Zinc Plating Works
Zinc plating is a process in electroplates or electrochemically coats a thin layer of zinc onto a metal surface. This layer of zinc acts as a barrier between the metal and its environment, helping to prevent corrosion caused by oxidation. The thickness of the coating depends on the application; for example, thicker coatings are often used for outdoor applications where there is more exposure to elements such as rain and moisture. Zinc plating also has aesthetic benefits; it can give metals a polished look and can even provide electrical conductivity if needed.
How Anodizing Works
Anodizing is another popular method of protecting metal from corrosion. This process involves immersing the metal piece in an electrolyte solution and then passing an electric current through it, which causes oxygen ions to form on the surface of the part in question. This creates an oxide film that helps protect against harsh environmental conditions like saltwater exposure or acidic soils. Anodized parts are also highly resistant to abrasion and wear, making them ideal for industrial applications where parts must endure heavy use or regular maintenance operations. Additionally, anodized parts are aesthetically pleasing and available in a wide range of colours depending on their application needs.
Difference Between Zinc Plating and Anodizing
- Zinc plating is a process in which a thin layer of zinc is applied to a metal surface in order to protect it from corrosion.
- Anodizing is a process in which an oxide layer is applied to a metal surface in order to protect it from corrosion.
- Zinc plating is typically less expensive than anodizing.
- Anodizing is typically more durable than zinc plating.
- Zinc plating can be used on a variety of metals, including steel, brass, and aluminium.
- Anodizing can only be used on aluminium.
Conclusion:
When it comes to protecting metals from corrosion, there are two main processes that you should consider: zinc plating and anodizing. Each offers unique advantages based on what type of protection you need for your product or project. Zinc plating provides a thin protective layer over a metal surface that prevents oxidation-related corrosion, while anodizing provides superior resistance to wear and tear along with aesthetic benefits like colour customization options. Consider both processes carefully before deciding which one is best for your needs!

Abhishek is a seasoned blogger and industry expert, sharing his insights and knowledge on various topics. With his research, Abhishek offers valuable insights and tips for professionals and enthusiasts. Follow him for expert advice on the latest trends and developments in the metal industry.