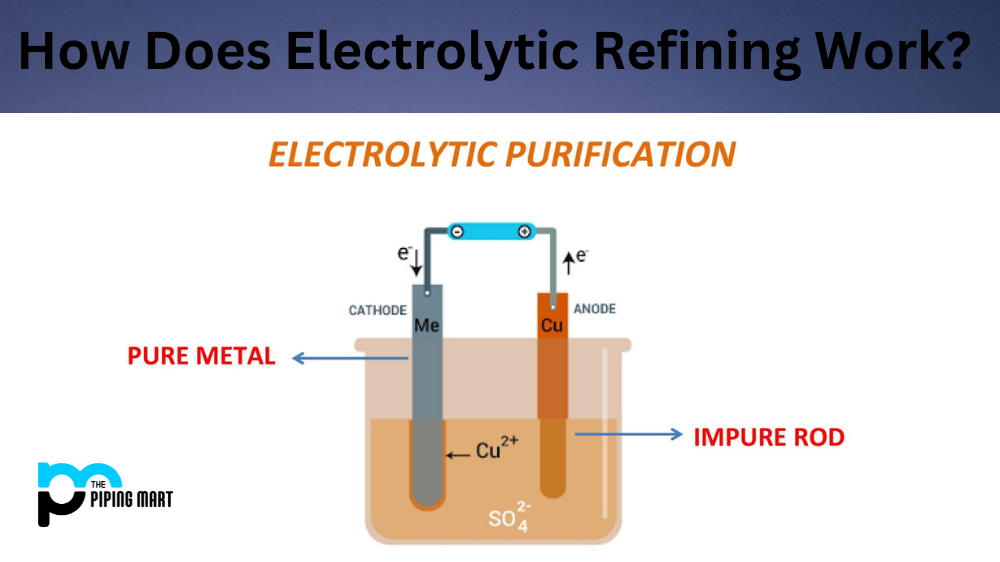
Electrolytic refining is a process used to purify and refine metals like copper, gold, and silver. This process involves passing an electric current through the metal in a particular electrolytic cell. As the current passes through the metal, impurities are removed, and purified metal is recovered from the bottom of the cell. In this article, we’ll take a closer look at how electrolytic refining works and why it’s so important for producing high-quality metals.
Process of Electrolytic Refining
The electrolytic refining process begins with a material containing impurities being placed into an electrolyte solution. An anode is placed into the solution as well, and this serves as a source of electrons for the reaction. When electricity is applied to the cell, positively charged ions travel toward the negative electrode (the cathode), while negatively charged ions travel toward the positive electrode (the anode). During this process, some of these ions are deposited onto the electrodes. These ions then form compounds with other materials in solution and can be further refined or purified using different methods.
Benefits of Electrolytic Refining
Electrolytic refining has many benefits that make it popular among metal producers. For one thing, it’s relatively inexpensive compared to other purification methods because it requires little energy input. Additionally, since it doesn’t generate any harmful byproducts or waste products, it makes for a much more sustainable process than some of its alternatives. Finally, because electrolysis can effectively remove impurities from metals without changing their core properties or composition, it’s a great way to produce high-quality products without having to invest in costly purification processes afterward.
Conclusion
Electrolytic refining is an effective method for purifying metals such as copper, gold, and silver by passing an electric current through them in a special electrolyte cell. Not only is this method relatively inexpensive compared to other methods, but it also produces no waste products or harmful byproducts and can effectively remove impurities without altering core properties or composition. All of these factors make electrolysis one of the most popular ways to produce high-quality metals today!

A passionate metal industry expert and blogger. With over 5 years of experience in the field, Palak brings a wealth of knowledge and insight to her writing. Whether discussing the latest trends in the metal industry or sharing tips, she is dedicated to helping others succeed in the metal industry.