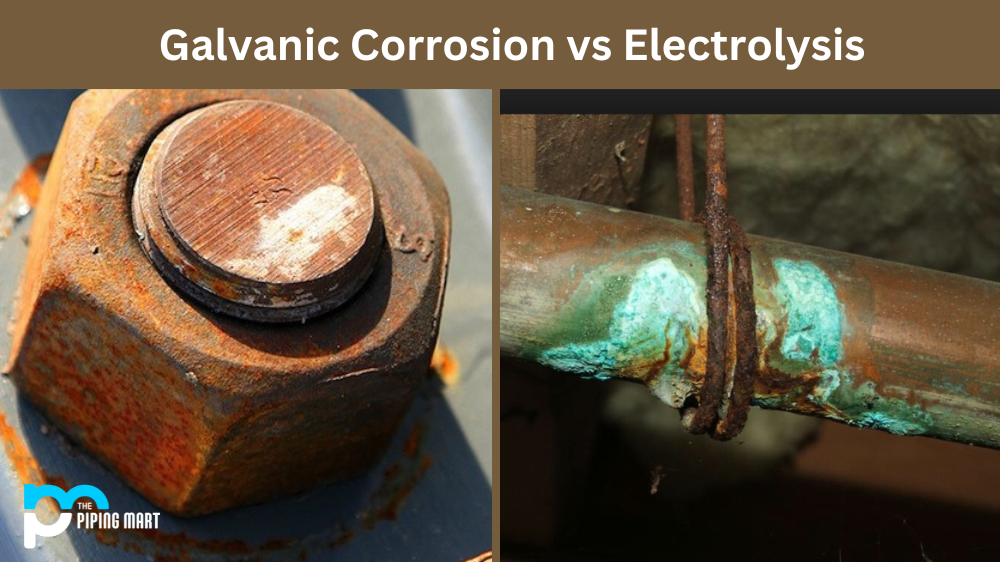
We often hear these two words used interchangeably, but what do they mean? Galvanic corrosion and electrolysis are similar processes when two dissimilar metals are submerged in a conductive solution. Understanding the differences between the two can help you better protect your metal components from damage.
What is Galvanic Corrosion?
Galvanic corrosion is a corrosion process caused by an electrochemical reaction between two dissimilar metals when they come into contact with each other in a conductive environment. This reaction causes one metal to corrode faster than it would naturally while its partner metal receives protection from further corrosion. A classic example of galvanic corrosion is when copper piping corrodes quickly when connected to steel or iron pipes in water systems.
When galvanic corrosion occurs, it results in an electric current which flows through the metal and the electrolyte (conductive solution), which causes an unequal distribution of ions across the surface of each metal. This leads to an accumulation of ions on one side of the metal, causing localized corrosion and weakening that area over time. The corrosive effect can be reduced by using appropriate material combinations and coatings such as paints or inhibitors.
What is Electrolysis?
Electrolysis is a process where electrical energy is used to drive chemical reactions. It works by passing an electrical current through two electrodes immersed in a liquid electrolyte solution, which causes chemical reactions at both electrodes. The resulting chemical compounds are then removed from the solution by either being deposited onto surfaces or dissolved into it. Electrolysis can be used to clean metals, weld metals together, and even create new alloys from different materials.
Unlike galvanic corrosion, electrolysis does not cause any permanent damage to metals; however, it can lead to some temporary warping due to heat build-up during welding operations or other types of processing that involve high levels of electrical current passing through the metal components involved. Additionally, because electrolytic processes may require large amounts of electricity for long periods, they can become costly if not appropriately managed. To reduce costs associated with this type of operation, manufacturers may opt for more efficient equipment designs that reduce power consumption and waste heat generated during processing operations.
Difference Between Galvanic Corrosion and Electrolysis
Anode
The anode is the electrode that is connected to the negative terminal of a battery or power source. In galvanic corrosion, the anode is the metal that corrodes. In electrolysis, the anode is the electrode that is used to pass the electric current through the solution.
Cathode
The cathode is the electrode that is connected to the positive terminal of a battery or power source. In galvanic corrosion, the cathode is the metal that does not corrode. In electrolysis, the cathode is the electrode used to collect the electrons generated by the reaction.
Electrolyte
An electrolyte is a substance that contains ions and can conduct electricity. The electrolyte is typically water or liquid containing dissolved minerals in galvanic corrosion. In electrolysis, the electrolyte generally is an acidic or basic solution that helps to conduct electricity.
Galvanic Series
The galvanic series is a list of metals in order of their reactivity with each other. The most reactive metal will corrode first when it comes into contact with another metal, while the least reactive metal will be protected from corrosion.
Conclusion:
Both galvanic corrosion and electrolysis have their advantages and disadvantages depending on how they’re applied; however, understanding how each process works can help you make more informed decisions about how best to protect your metal components from unnecessary damage caused by either process over time. Ultimately, choosing the suitable materials combined with proper maintenance and inspections can ensure your equipment remains in top working condition for years to come!

Meet Bhavesh, a seasoned blogger with a wealth of knowledge and experience. From metal products manufacturing to retail, Bhavesh has a diverse background in various industries and is dedicated to sharing his insights and expertise with readers.